知识分享|EMA药品、活性物质、辅料和内包装灭菌指南翻译与解读(三)
4.1.6. Aseptic processing
无菌工艺
Aseptic processing is not considered to be a sterilisation process but concerns the usage of technologies to process sterile components avoiding addition of microbiological contaminants, e.g. use of an isolator or Restricted Access Barrier System (RABS).
无菌工艺不被认为是灭菌过程, 但涉及使用对无菌组分进行处理的技术,以避免引入微生物污染,例如使用隔离器或隔离系统 (RABS)。
For aseptic processing, information on the bulk holding time before filling and on the filling time should be stated and appropriately supported by data. The times should be minimised. The grounds for holding and filling times longer than 24 hours should be justified and supported by a risk assessment. It should be verified that the results of the media simulations support the proposed holding and processing times. The actual results of media simulations fall within the field of GMP and need not be presented routinely, but may be requested by the competent authorities in certain circumstances since such data are important to justify proposed holding and filling times.
对于无菌工艺, 应说明灌装前的保存时间和灌装时长的信息, 并有适当的数据支持。时间应尽量减少。保存时间和灌装时间超过24小时的理由应经过论证并有风险评估支持。应确认培养基模拟灌装的结果能够支持保存时间和灌装时间。培养基模拟灌装的实际结果属于GMP领域,不需要例行提出,但在某些情况下, 主管当局可能会提出要求, 因为这类数据对于证明拟议的保存时间和灌装时长的合理性是很重要的。
Sterile containers should be used for aseptically treated active substances, excipients and finished products.
经无菌处理的活性物质、辅料和成品应使用无菌容器。
Where blow-fill-seal technology is used for aseptically treated products, a summary of the validation data should be provided to confirm that the container produced is sterile. The validation should, using a biological indicator with a suitable resistance, demonstrate a SAL of ≤10-6 for the surface of the container. The bioburden of the material(s) used for the manufacture of the blow-fill-seal container should be controlled. The limit should be justified in relation to the lethality of the validated blow-fill-seal process. The bioburden limit should also include a safety margin as a for any possible bioburden enclosed within the material.
当吹-灌-封技术用于在无菌处理产品时,应提供验证数据总结, 以确认所使用的容器是无菌的。验证应使用具有适当耐热性的生物指示剂, 证明容器表面的 SAL≤10-6。应控制用于制造吹-灌-封容器的材料的生物负荷。生物负荷的限度应结合吹-灌-封工艺的杀灭力进行论证。生物负荷限度还应包括安全系数,以防止材料中包含的任何可能的生物负荷。
The majority of ATMPs cannot be terminally sterilised. In such cases, the manufacturing process should be conducted aseptically. Further details on aseptic manufacturing for ATMPs can be found in the Guidelines on Good Manufacturing Practice for Advanced Therapy Medicinal Products.
大多数ATMP不能进行最终灭菌。在这种情况下, 生产过程应无菌。有关 ATMP无菌生产的更多详细信息,请参见“先进治疗药品良好生产规范指南”。
4.2. Good manufacturing practice for sterile active substances, sterile excipients and sterile containers
无菌活性物质、无菌辅料和无菌容器GMP
Volume 4 of "The rules governing medicinal products in the European Union" contains guidance for the interpretation of the principles and guidelines of good manufacturing practices for medicinal products for human and veterinary use laid down in Commission Directives 91/356/EEC, as amended by Directive 2003/94/EC, and 91/412/EEC respectively. For Advanced Therapy Medicinal Products, the Guidelines on Good Manufacturing Practice specific to Advanced Therapy Medicinal Products should be followed.
“欧盟药品管理法”第4卷包含了经第2003/94/EC号指令和第91/412/EEC号指令修正的第91/356/EEC号委员会指令中规定的人用和兽用医药产品GMP的原则和准则的解释指南。对于先进治疗药品, 应遵循“先进治疗药品的良好生产规范指南”。
4.2.1. Active substances
活性成分
The basic GMP requirements for active substances used as starting materials (European Union (EU) GMP guide part II) apply to the manufacture of sterile active substances up to the point immediately prior to the active substance being rendered sterile. The sterilisation and aseptic processing of sterile active substances are not covered by EU GMP Part II but should be performed in accordance with the principles and guidelines of GMP as laid out in the relevant EU Directive and interpreted in the GMP Guide including its Annex 1.
作为起始物料的活性物质的基本GMP要求 (欧盟 (EU) GMP指南 第二部分 ) 适用于无菌活性物质在进入无菌步骤之前的生产。无菌活性成分的灭菌和无菌处理不在欧盟 GMP 第二部分的范围内, 但应按照欧盟相关指令中的规定和 GMP指南中解释的GMP 原则(包括其附录1)和指南进行。
The sterilisation and aseptic processing of active substances is considered to be a step in the manufacture of the medicinal product. This implies that for any active substance manufacturer who performs sterilisation and subsequent aseptic handling of the active substance, a valid manufacturing authorisation or GMP certificate from an EEA authority or from an authority of countries where mutual recognition or other Community arrangements apply has to be submitted.
活性物质的灭菌和无菌加工被认为是药品生产的一个步骤。这意味着,对活性物质进行灭菌及随后无菌处理的任何活性物质生产商,需要提交EEA药监局,或互认的国家药监局颁发的或社会团体安排申请的有效的生产许可或GMP证书。
The same GMP and data requirements also apply to sterile active substances supported by a Certificate of Suitability issued by the European Directorate for the Quality of Medicines & HealthCare (EDQM) or described in an Active Substance Master File (ASMF).
同样的GMP 和数据要求也适用于由EDQM颁发的符合性证书(COS)支持的无菌活性物质, 或在活性物质主文件 (ASMF) 中描述的无菌活性物质。
4.2.2. Excipients
辅料
All the excipient sterilisation sites should be stated by name and address in the dossier.
所有的辅料灭菌工厂都应在注册文件中注明名称和地址。
For excipients required to be sterile (i.e. those subsequently used in an aseptic manufacturing process), the site where sterilisation of the excipients takes place may not have undergone inspection by an EU authority and consequently may not hold an EU GMP certificate in relation to this activity. Nevertheless the sterilisation of an excipient is a critical process and the sterility of the excipient is a critical quality attribute to ensure the sterility of the finished product. When a GMP certificate is not available, a statement should be provided confirming that the finished product manufacturer has evaluated all the manufacturers of sterile excipients with regards to their quality system related to the sterilisation of the excipient. For products for human use this evaluation should be conducted in line with the (GMP) Guidelines of 19 March 2015 on the formalised risk assessment for ascertaining the appropriate good manufacturing practice for excipients of medicinal products for human use by taking into account the specific requirements of Annex 1 of EU GMP-Guidelines.
对于需要无菌的辅料 (例如,直接用于无菌生产过程的辅料), 对辅料进行灭菌的工厂可能没有经过欧盟当局的检查, 因此可能无法持有与此活动相关的欧盟GMP证书。然而, 辅料的灭菌是一个关键的过程, 辅料的无菌性是确保成品无菌的一个关键的质量属性。在没有GMP证书的情况下, 应提供一份声明, 确认成品生产商已对所有无菌辅料生产商进行了与辅料灭菌相关的质量体系评价。对于人用药品,这一评价应按照2015年3月19日发布的关于正式风险评估的 (GMP) 指南进行,考虑欧盟GMP附录1的具体要求,以确定所适用的人用药品辅料的GMP。
4.2.3. Containers
容器
For containers required to be sterile (i.e. those subsequently used in an aseptic manufacturing process), the site where sterilisation of the containers takes place may not have undergone inspection by an EU authority and consequently may not hold an EU GMP certificate in relation to this activity1. When a GMP certificate is not available, certification that the sterilisation has been conducted and validated in accordance with the following ISO standards would be considered sufficient to provide an acceptable level of sterility assurance for the empty container:
对于需要无菌的容器 (例如直接用于无菌生产的容器),对容器进行灭菌的工厂可能没有经过欧盟当局的检查, 因此可能没有持有与此活动相关的欧盟GMP证书。在没有GMP证书的情况下, 证明已按照以下 ISO标准进行灭菌和验证,将被视为足以提供适当无菌保证水平的容器:
1.I.S. EN ISO 20857 Sterilization of Health Care Products - dry Heat - Requirements for the Development, Validation and Routine Control of a Sterilization Process for Medical Devices;
2.I.S. EN ISO 11135 Sterilization of Health-care Products - Ethylene Oxide - Requirements for the Development, Validation and Routine Control of a Sterilization Process for Medical Devices;
3.I.S. EN ISO 17665-1 Sterilization of Health Care Products - Moist Heat - Part 1: Requirements for the Development, Validation and Routine Control of a Sterilization Process for Medical Devices, and, ISO/TS 17665-2 Sterilization of health care products -- Moist heat -- Part 2: Guidance on the application of ISO 17665-1;
4.I.S. EN ISO 11137-1 Sterilization of Health Care Products - Radiation - Part 1: Requirements for Development, Validation and Routine Control of a Sterilization Process for Medical Devices;
5.I.S. EN ISO 11137-2 Sterilization of Health Care Products - Radiation - Part 2: Establishing the Sterilization Dose;
6.I.S. EN ISO 11137-3 Sterilization of Health Care Products - Radiation - Part 3: Guidance on Dosimetric Aspects.
It is the responsibility of the manufacturer of the medicinal product, to ensure the quality, including sterility assurance, of containers. The site where QP certification of the finished product takes place, and other manufacturing sites which are responsible for outsourcing this sterilisation activity, should have access to the necessary information to demonstrate the ongoing qualification status of suppliers of this sterilisation service. This may be checked during inspections of the manufacturer of the finished product. The Competent Authorities may also decide, based on risk, to carry out their own inspections at the sites where such sterilisation activities take place.
确保容器的质量,包括无菌保证是药品生产商的责任。对成品进行QP的工厂,以及负责将这一灭菌活动外包的其他制造工厂, 应能获得必要的信息, 以证明此灭菌服务供应商的持续确认状态。这可能会在成品生产商的检查过程中进行检查。主管当局还可根据风险决定对进行此类灭菌活动的工厂进行自己的检查。
Quality Dossier requirements
质量档案要求
The following details regarding the sterilisation of the container components should be included in the quality dossier:
关于容器部件灭菌的以下细节应包括在质量档案中:
1.The sterilisation method and sterilisation cycle;
灭菌方法和灭菌周期
2.Validation of the sterilisation cycle if the sterilisation cycle does not use the reference conditions stated in the Ph. Eur.;
灭菌周期的验证,如果灭菌周期不使用 Ph. Eur 中规定的参考条件;
3.The name and address of the site of sterilisation and, where available*, details of GMP certification of the site.
灭菌场地的名称和地址, 以及该场地 GMP 证书的详细信息(如适用) *。
*Where the container component is a CE-marked Class Is sterile device (e.g. sterile syringe), a declaration from the device manufacturer that the component is a Class Is sterile device, together with a copy of the certificate of conformity from the Notified Body will suffice. In the absence of a GMP certificate or declaration that the component is a CE-marked Class Is medical device, confirmation by finished product manufacturer that the sterilisation process has been conducted and validated in accordance with the relevant ISO standards should be provided.
* 如果容器组件是 CE标志等级I的无菌器械 (例如无菌注射器),则一份来自该器械生产商声明该组件是等级I无菌器械的声明,以及认证机构提供的符合性证书副本,足够。在没有 GMP 证书或声明组件是CE标志等级I 医疗器械的情况下, 应提供成品制造商确认已按照相关 ISO 标准灭菌和验证的证明。
【解读】
这部分内容要求是无可非议的,国内很多企业基于客户的要求,会去做无菌原料药出口,从本指南可以清楚的看出,实际无菌原料药的要求和无菌制剂的要求就无菌保证这部分已经没有任何的区别了,一个做原料药的企业,要承担和无菌制剂企业一样的GMP体系维护成本去满足客户的要求,那么做老板的要好好考量下客户出的价格是否真的可以涵盖这部分的成本了。
对于辅料和容器而言,更多的是提醒那些计划进行无菌制剂出口的企业,对自己的这些物料的供应商的监管和现场审计的标准。目前国内企业供应链的管理还没有提升到欧美标准的水平,既没有合格的专人也没有足够的资源配制,而国内供应商的实际状态也不能符合欧美GMP的基本要求,所以这个部分又成了我们的软肋。
4.3. Selection of sterilisation method
灭菌方法的选择
Finished products intended to be sterile should be terminally sterilised in their final container whenever possible, as clearly stated in the Ph. Eur., general chapter 5.1.1. Similarly, active substances, excipients and containers when required to be sterile should be packed before they are sterilised whenever possible. When terminal sterilisation by heat is not possible, the application of an alternative method of terminal sterilisation, sterilising filtration and/or aseptic processing may be considered. It is recognised that terminal sterilisation processes utilising conditions other than the Ph. Eur. reference conditions may be developed to provide satisfactory SALs and such alternative processes may be acceptable when properly designed, validated and controlled.
欧洲药典通论5.1.1 中明确指出, 无菌成品应尽可能在其最终容器中进行最终灭菌。同样, 需要无菌时, 活性物质、辅料和容器应包装好,然后尽可能进行灭菌。当不能用热进行终端灭菌时, 可以考虑采用一种替代终端灭菌的方法,如除菌过滤和/或无菌工艺。开发使用欧洲药典参考条件以外的其他终端灭菌工艺,以提供令人满意的SAL也是被认可的,这种替代工艺如经适当设计、验证和控制是可以接受的。
If a sterilisation process using principles other than those described in the Ph. Eur. (steam, dry heat, ionising radiation, gas sterilisation and sterilising filtration) is intended to be used for the sterilisation of an active substance, excipient, container or finished product, the applicant may consider seeking scientific advice regarding the acceptability of the method and the documentation required.
如果打算使用欧洲药典中(蒸汽、干热、电离辐射、气体灭菌和除菌过滤)以外的其他灭菌方法对活性物质、辅料、容器或成品进行灭菌,申请人可考虑就该方法的可接受性和所需文件寻求科学意见。
During the manufacturer’s evaluation of whether a terminal sterilisation cycle is possible, substantial efforts should be made to enable terminal sterilisation. If the active substance or another component of the finished product is shown to degrade significantly or an impurity limit is exceeded during shelf-life under even the least stressful terminal sterilisation conditions, the efforts made to develop a formulation and container capable of undergoing terminal sterilisation should be presented in the development section. Such efforts could be selection of optimal pH, choice of excipients (qualitative and quantitative), container, optimisation of sterilisation method and manufacturing conditions.
在生产商评估是否有可能进行最终灭菌的过程中,应尽可能使用终端灭菌。如果即使在最缓和的最终灭菌条件下, 活性物质或成品的其他成分在其有效期内仍会明显下降或杂质超标,则应在研发部分应说为开发耐受最终灭菌工艺的配方和容器所做的努力,包括选择最佳 ph 值、选择辅料 (定性和定量)、容器、优化灭菌方法和生产条件。
In case of medicinal products containing highly sensitive active substances, (e.g. proteins or other heat labile biological substance), where it is well known that terminal sterilisation is not possible, a justification based on a scientific rationale is generally acceptable and further justification of the choice of aseptic processing discussed later in section 4.3 may not be needed.
如果是含有高敏感性的活性物质 (如蛋白质或其他热不稳定的生物物质) 的医药产品, 众所周知, 不可能进行最终灭菌, 基于科学合理的论证通常是可以接受的,不需要进一步论证选择第4.3 节无菌工艺的理由。
The principles for the choice of sterilisation process for finished products and containers are presented in the form of decision trees in section 5 of this guideline. The principles of the decision trees may also be applied for the sterilisation of active substances and excipients.
本指南第5节以决策树的形式介绍了成品和容器灭菌工艺选择的原则。决策树的原则也可适用于活性物质和辅料的灭菌。
For finished products where terminal sterilisation is not possible and aseptic processing is proposed, the decision trees should be applied to individual components or mixtures of components in the formulation. An impact on the shelf-life or storage conditions caused by a terminal sterilisation process is not in itself a reason to exclude terminal sterilisation, unless the new storage condition or shelf-life would cause significant problems for the user.
对于不可能进行最终灭菌并拟进行无菌处理的成品,决策树应应用于配方中的单个组分或组分的混合物。最终灭菌过程对有效期或储存条件造成的影响本身并不是排除最终灭菌的理由, 除非新的储存条件或有效期会给用户带来重大问题。
Terminal sterilisation should not be ruled out purely on the basis of an increase in degradation products above the qualification thresholds in ICH Q3A/VICHGL10 (active substances), ICH Q3B/ VICH GL11 (finished products) or the impurity limits in ICH M7 for products in the scope of that guideline without additional justification. If impurities are either metabolites or are generated at levels already qualified, then terminal sterilisation is still considered feasible. However, if the degradation products are not qualified at the level at which they occur, then sterile filtration and aseptic processing may be selected. For medicinal products for human use impurities which occur above the identification threshold should be specified in the finished product specification.
不应仅仅因为降解产物增加超过 ICH Q3A/VICHGL10 (活性物质)、ICH Q3B/VICH GL11 (成品) 中的限度或ICH M7指导范围中的产品的杂质限度,就排除最终灭菌而不进行论证。如果杂质都是代谢产物或是产生的杂质在确认水平, 那么最终灭菌仍然被认为是可行的。然而, 如果产生的降解产物的水平不在其确认水平, 则可以选择除菌过滤和无菌工艺。对于人用药产品, 超过鉴别阈值的杂质应在成品标准中明确规定。
The risk induced by the degradation should be balanced by the risk induced with an aseptic manufacturing method, also taking in account the posology of the finished product and the nature of the degradation products. Attempts to find terminal sterilisation conditions adjusted to give acceptable impurity levels based on degradation mechanisms of the active substance and the actual bioburden should be described in the quality dossier.
降解引入的风险应与无菌生产方法引入的风险进行平衡, 同时考虑到成品的剂量和降解产物的性质。在质量档案中, 应说明根据活性物质的降解机制和实际生物负荷,调整最终灭菌条件,得到可接受的杂质水平而进行的尝试。
【解读】
这部分更加实际的解释了在选择“最终灭菌”还是“无菌工艺”的区别和思考点,尤其是对降解杂质的限度考量给出了允许超出相关指南的条件。这里的灵活性基于的原则还是已知降解杂质的风险大小和无菌性能的风险大小的权衡。这个思考点和优先等级的处置,值得我们关注和在技术开发的过程中先期导入。
In certain cases, as described in the bullet points below, the use of aseptic processing may be accepted, even if the formulation itself can be terminally sterilised. The approach should be clearly documented, explained and scientifically justified. Such cases could be justified by:
在某些情况下, 如下文要点所述, 即使配方本身可以最终灭菌, 也可以使用无菌工艺。应明确记录、解释和科学论证这一方法。这种情况的通过以下方式论证:
不能最终灭菌的容器有利于用户利益, 例如:
能实现单滴液滴滴到眼睛里的滴眼容器;
能实现非注射用多剂量无防腐剂的人用药品的容器;
更易服用;
更安全地处理有毒药品,例如细胞毒性药品使用塑料瓶而不是玻璃瓶。
选择使用热不稳定容器本身不能成为不采用最终灭菌工艺的唯一理由,应研究使用替代材料。因此,应包含关于研究可以最终灭菌的容器的研究的讨论。
为有效期少于一周的放射性药物产品提供尽可能长的保质期。
【解读】
这部分的两个情形,天平又向无菌工艺倾斜,原因是显而易见的,微生物的潜在危害是可控的,是和产品的效应和病人的其他应用危害进行对比的选择。
无菌工艺的可接受性应基于决策树的应用和风险评估。下面的要点并非旨在用于证明无菌工艺的合理性,而仅旨在为评估灭菌或无菌工艺的可接受性时需考虑的问题提供指导。考虑因素包括(但不限于):
推荐的容器可以提高用户利益的证据;
活性物质、灭菌过程中产生的杂质的降解机理和毒性的稳定性;
每剂给药的体积
总之,所选择的灭菌或无菌工艺的理由应包括详细的利益风险评估,并且应该证明已经进行了适当的研发工作。
对于先进的治疗药品,当成品不能灭菌时,所有组分的微生物学质量,工艺设备和生产工艺的无菌技术都是至关重要的。对于那些不能灭菌的药品,如基于细胞的药品,应提供有关微生物污染的详细风险评估。对这些ATMP产品,基于风险的方法已经被预见到。(参见基于风险的方法指南,根据适用于高级治疗药物的指令2001/83 / EC的第IV部分附录I,EMA / CAT / CPWP / 686637/2011)。
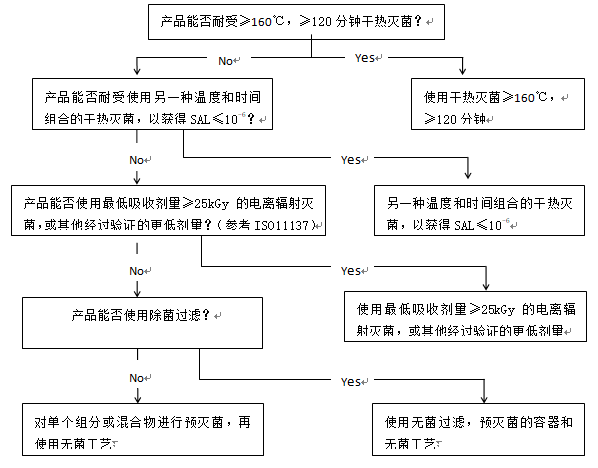
5. Decision trees
决策树
图1和图2中的决策树旨在帮助选择最佳的灭菌方法,同时考虑到需要考虑的各种问题。决策树越向下的方法,无菌保证越低, 因此, 通常应选择第一个可行的选择*. 决策树主要针对含有化学活性物质的成品进行了详细阐述,但也可适用于其他类型的产品(包括活性物质和辅料)。图3提供了空容器的相应信息。决策树不适用于有CE标记的医疗器械的无菌空容器。对于生物产品,可采用替代办法。
虽然通过加热灭菌和通过电离辐射灭菌提供了同样的无菌保证, 但加热灭菌的风险低(辐射杂质更加复杂),也比电离辐射灭菌更容易控制。因此, 在决策树中, 加热灭菌优先于电离辐射灭菌。
【解读】
这部分和之前没有显著性的区别,没有更多值得讨论和解读的地方。
图1 对于溶液剂型产品灭菌方法选择的决策树:
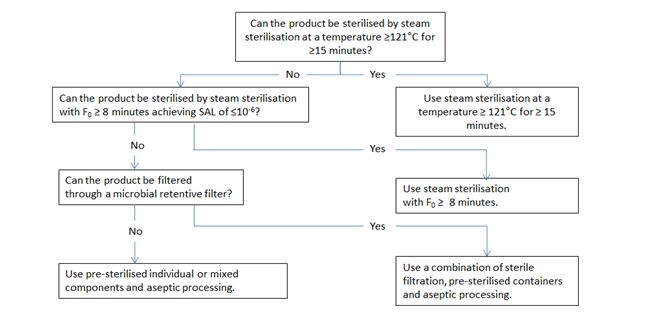
图2 干粉产品,非溶液剂型和半固体产品灭菌方法选择的决策树:
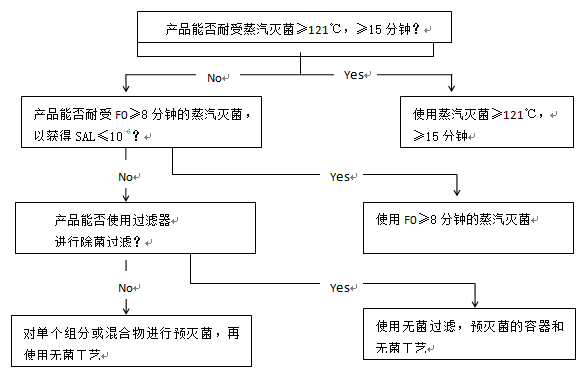
Figure 3 Decision tree for sterilization choices for containers
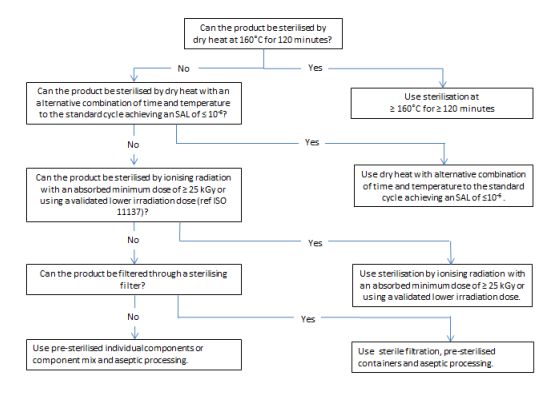
图3 容器灭菌工艺选择的决策树

全部 0条评论